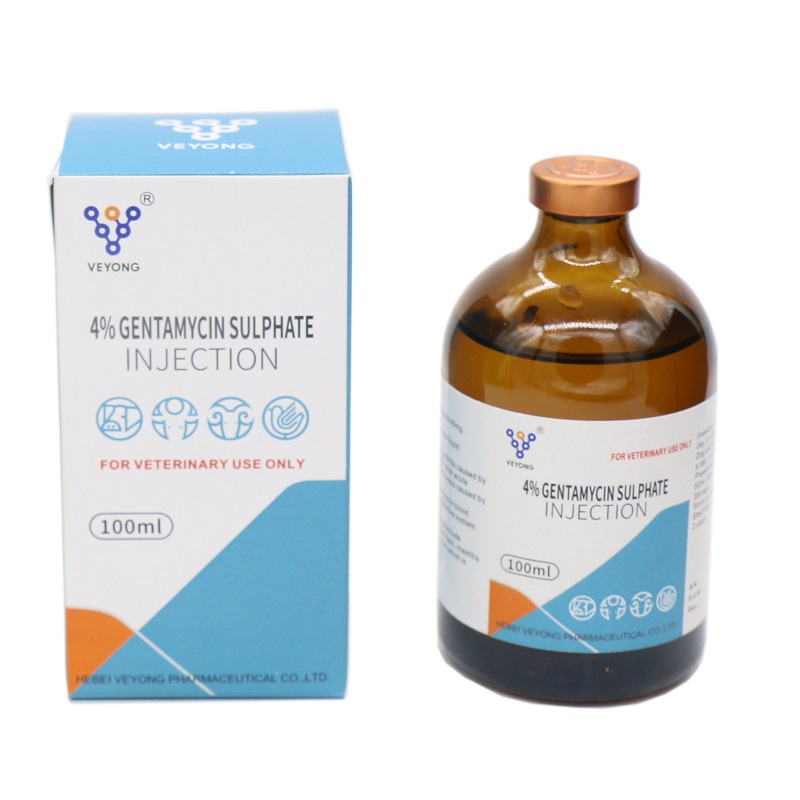
IV% Gentamyclin sulphate iniectio
Indicium
Species:Hoc productum est hyalinae ad flavo vel flavo viridi liquidum liquidum.
Pharmacological actio:PharmacodynamicGentamycinis an aminoglycoside antibiotic with antibacterial effect on a variety of gram-negative bacteria (such as Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Pasteurella, Salmonella, etc.) and Staphylococcus aureus (including β-lactamase-producing strains). Most Cocci (Streptococcus pyogenes, Pneumococcus, Streptococcus Faecalis, etc.), anaerobic bacteria (Bacteria et Clostridium), Mycobacterium tuberculosis, Rickettsia et fungi sunt repugnant ad hoc productum.
Pharmacokinetics:Effusio est celeri et integrum post intramuscular iniectio. Peccare concentrationes sunt pervenit in 0,5 ad I hora. Bioavailability excedit XC% ad telae vel intramuscular iniectio. Est maxime excernitur per glomerular filtration et rationes ad XL% ad LXXX% of the administratum dose. The elimination half-life after intramuscular injection is 1.8 to 3.3 hours in horses, 2.2 to 2.7 hours in calves, 0.5 to 1.5 hours in dogs and cats, 1 hour in cows and pigs, 1 to 2 hours in rabbits, and 2.3 to 3.2 hours in sheep, buffaloes, cattle, and dairy goats.
Medicamento interactiones:
(I) compositum de gentamycin cum tetracycline et Erythromycin potest habere contrahit.
(II) In tandem cum Cephalospistinins, dextran, potens urinam (ut Furosemide, etc.) et Erythromycin, ototoxicity huius uber potest esse aucta.
(III) Osseus musculus relaxants (ut succinymlcholine chloride, etc.) vel medicinae hoc effectum potest augendae in neuromuscular obturans effectus humira.
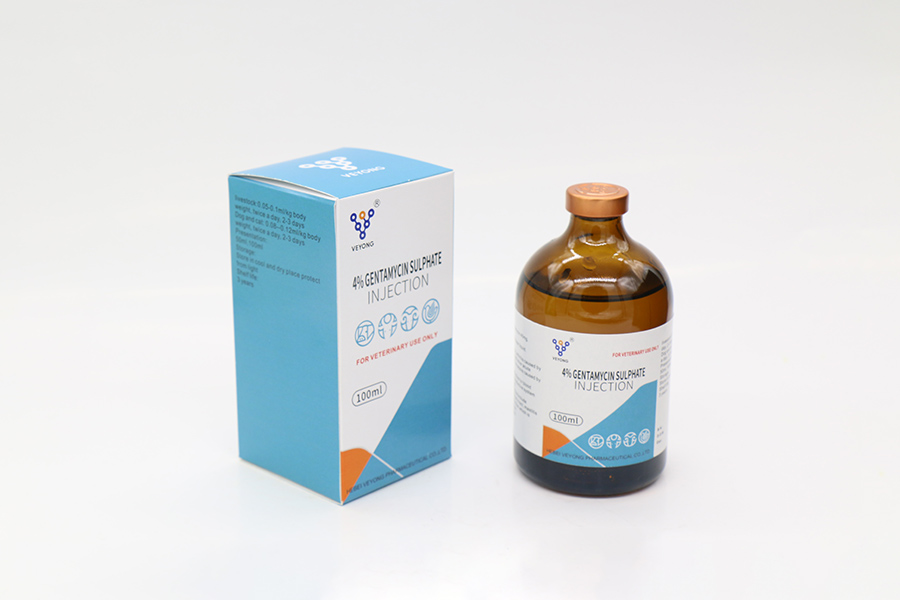
Actum et usum
Aminoglycoside antibiotics. Nam gram-negans et positivum bacterial infectiones.
Dosis et Administration
(I) ototoxicity. Saepe causat vestibulares damnum in aure, quod potest aggravari cum cumulus continenter administretur medicinae in dose-dependens modo.
(II) interdum allergic profectae. Feles sunt sensitivo, constans potest facere nausea, vomitus, salivam et ataxia.
(III) High doses potest facere neuromuscular conduction obsidionem. Accidentales mortes saepe fieri post General Anesthesia ad chirurgicam ratio in canibus et feles, cum penicillin ne contagione.
(IV) potest facere reversible nephrotoxicity.
Cautiones
(I) gentamycin potest esse in iunctura cum β-lactam antibiotics tractare gravibus infectiones, sed non repugnat cum mixta in vitro.
(II) In iunctura Penicillin, hoc productum habet synergistic effectus in Streptococci.
(III) Non habet respiratoriorum demissionem et non infunduntur intravenously.
(IV), ut contingat cum tetracycline et Erythromycin.
(V) combination cum Cephalosporins potest augendae nephrotoxicity.
Recessum tempus
Pig, bos et ovis ad XL dierum.
Repono
Signatum et condita in a frigus tenebris est.
Hebei veyong pharmaceutical Co., Ltd, erat statutum in MMII, sita in Shijiazhuang urbem, Hebei provinciam, Sina, deinde ad caput Beijing. Ea est magna GMM-certified VETERINARIUS ENTERIPRISE, cum R & D, productio et Sales VETERINARIUS APIS, Praeparata, premixed feeds et feed additives. Ut Provincialis Technical Center, Veyong constituit in novam R & D System pro nova VETERINARIUS medicamento, quod est nationaliter notum technicae innovation secundum VETERINARIUS Enterprise, sunt LXV technica professionals. Veyong habet duas productionem bases: Shijiazhuang et Ordos, de quibus Shijiazhuang basi opercula in area 78.706 M2, cum XIII API, et XI Production Productio, Premix, PRESTATUS, Bolus, Pesicid disinfectant, rectae. Veyong praebet apis, magis quam C own- label apparatu, et OEM & ODM Service.
Veyong attaches magni momenti ad administratione EHS (environment, salutem & salus) systema, et adeptus est ISO14001 et Ohsas18001 testimoniales. Veyong est enumerantur in opportuna emergentes Industrial conatibus in Hebei provinciae et can continua copia products.

Veyong statutum est completum qualitas administratione ratio, adeptus est ISO9001 Certificate, Sinis GMP Certificatorium, Australia APVMA GMP CEP CEP certificatorium, et US FDE. Veyong habet professional quadrigis de Registerationis, Sales et technica Service, nostri comitatu lucrata confidunt et subsidium a numerosis customers per optimum productum qualis, summus qualitas pre-venditio et post-venditionesque servitium, gravi et scientific administratione. Veyong fecit diu terminus cooperante cum multis internationally nota animal pharmaceutical conatibus cum products exportata ad Europam, Meridionalis America, Medius Oriente, Africa et regiones, etc.


.png)
.png)
.png)
.png)